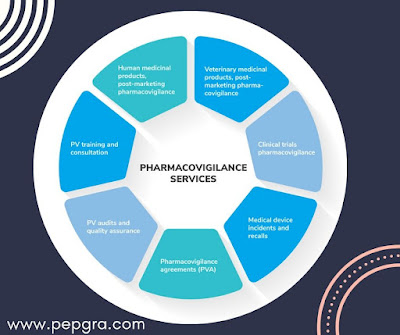
What is pharmacovigilance?

Pharmacovigilance also Known as Drug Safety, is that the Pharmacovigilance services concerning the gathering , detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products. As such, pharmacovigilance heavily focuses on adverse drug reactions, or ADRs, which are defined as any response to a drug which is noxious and unintended, including lack of efficacy. The condition, that this definition only applies with the doses normally used for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological disorder function was excluded with the foremost recent amendment of the applicable legislation Medication errors like overdose, and misuse and abuse of a drug also as drug exposure during pregnancy and breastfeeding, also are of interest (even without adverse event itself), because they'll end in an ADR. Information received from patients and healthcare providers via phar...