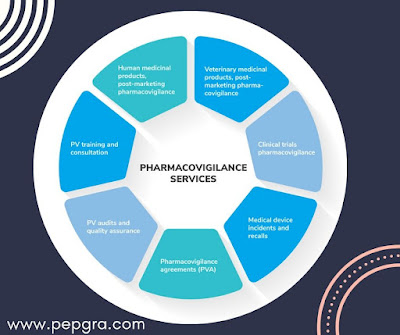
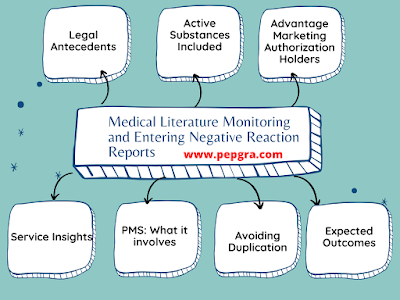
Pharmaceutical Legislation On Notice To Applicants And Regulatory Guidelines For Medicinal Products For Human Use | Pepgra

Notice to Applicants The European Commission in tandem with proficient authorities from the Member States, the European Medicines Agency along side interested parties created the Notice to Applicants (NTA). This was through with the target to fulfil the obligations of the commission with regards to article 6 of Regulation ( European Union ) No. 726/2004, and with regards to the Annex I to Directive 2001/83/EC as per the amendments. one among the primary editions of the Notice to Applicants (which is volume 2 within the series “The Rules governing medicinal products within the ecu Union) was initially published within the year 1986. This notice was subject to further revisions following which a completed version was issued i.e., the second edition was published in 1989(European Commision, 2020). Later, during 1993, the processes associated with applications for marketing authorizations were modified and therefore the mutual also as centralized regulation procedures came into...