Post Authorization Studies for Clinical Research Purposes - Pepgra Healthcare

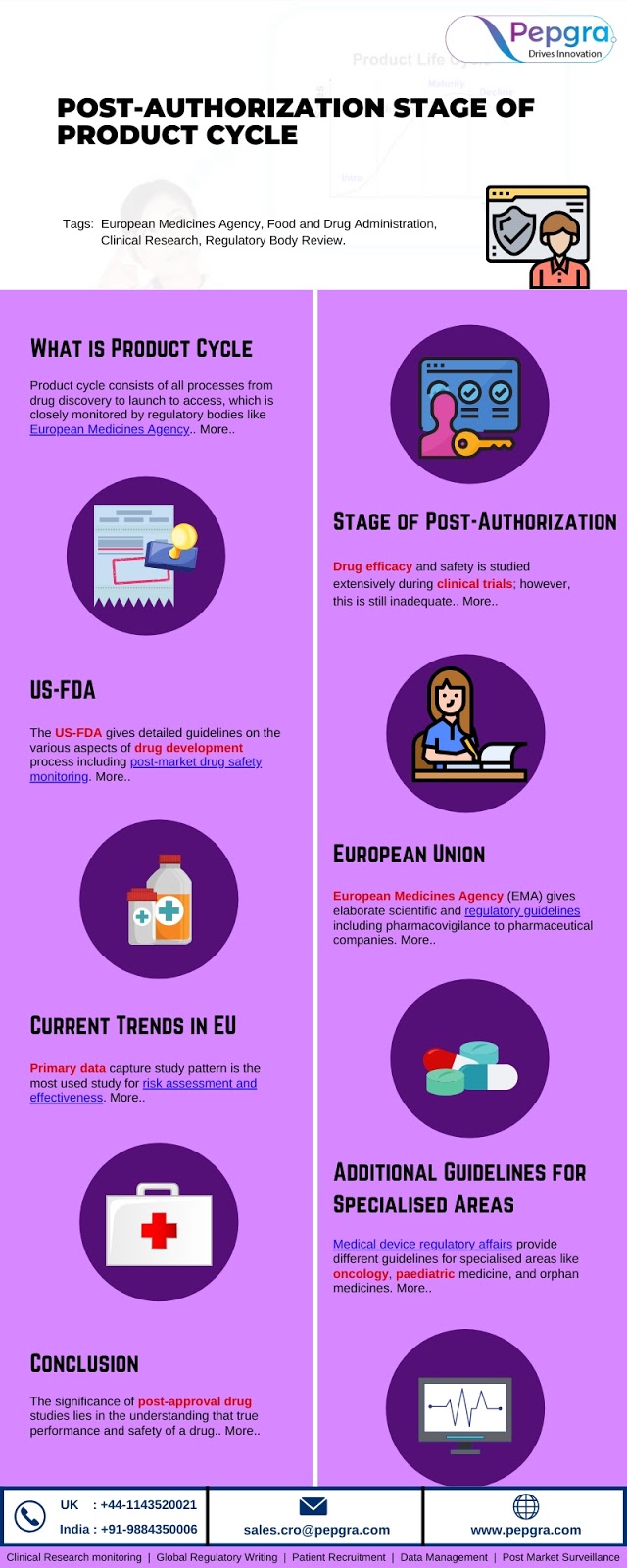
A PASS is an authorization of a study carried out after a medicinal product, to obtain further information on a medicinal product's safety or to measure the effectiveness of risk-management measures. Some changes which has been held in PASS are: 1. Transparency 2. Methodology 3. Definitions 4. Governance Continue Reading: http://bit.ly/3i1wrfe Contact us: UK: +44-1143520021 US/Canada: +1-972-502-9262 India: +91-9884350006 Email id: sales.cro@pepgra.com Website: www.pepgra.com